
150
METTLER TOLEDO
Industrial Catalog 2015
Product Inspection
Track & Trace and Quality Control
Pharmaceutical Packaging Industry
As a leading supplier of inline vision inspection solutions for the pharmaceutical in-
dustry, PCE provides comprehensive solutions for track and trace, serialization and
aggregation tasks and vision inspection equipment for packaging lines.
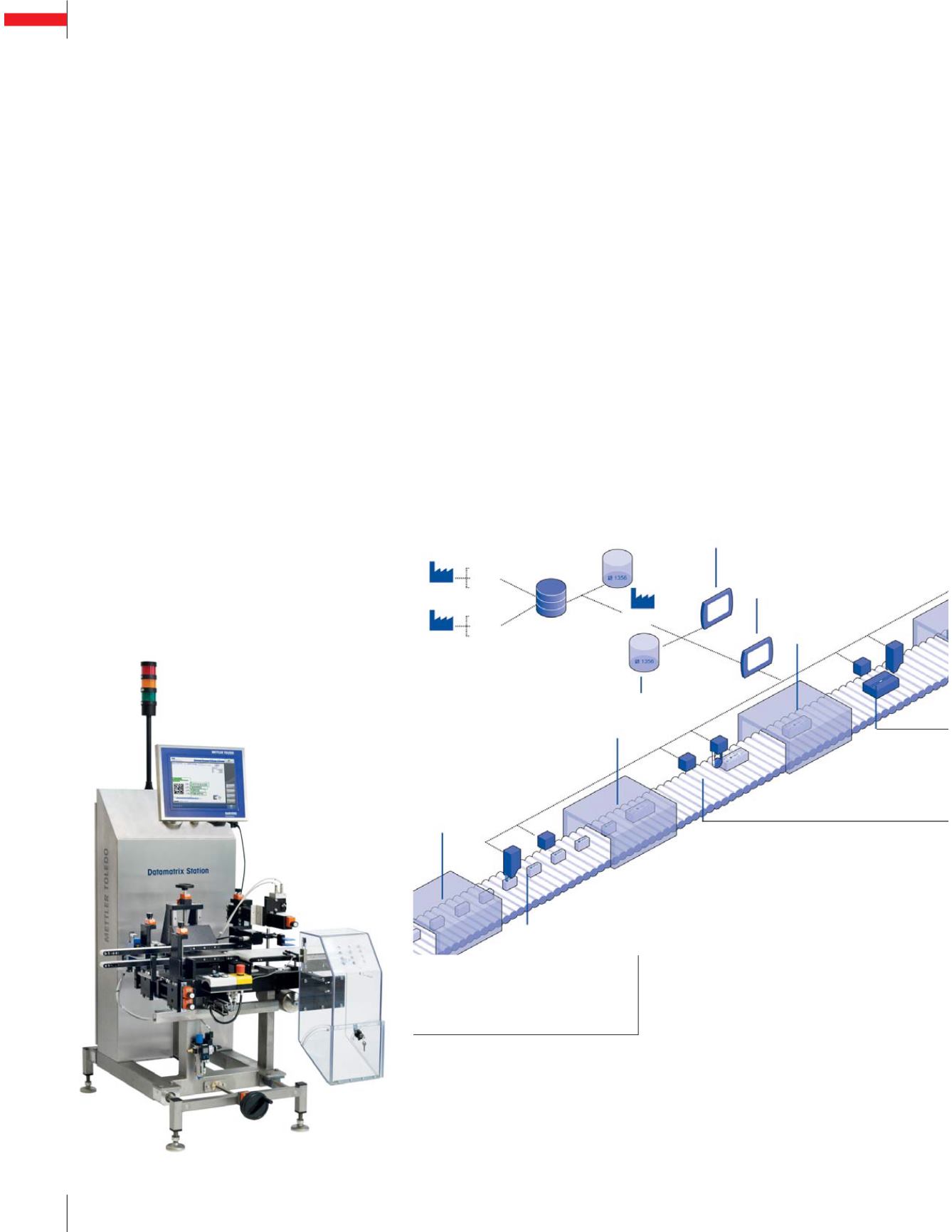
Manager and Pilot Site Manager)
ensures that printing, labeling and
verification of serialized data is
performed correctly. The PCE Pilot
Systems are available to mark
packs with individual serial num-
bers for full traceability of the
production or packaging process
and collect critical correspond-
ing process data. Solutions are
available for all packs including
single cartons to shipping cases
and ready-to-ship pallets. The PCE
management software (Pilot Line
software suite supports dedicated
devices as well as data exchange
to higher level IT systems.
Line 1
Line 2
Line 3
Line 1
Line 2
Line 3
Site 2
Cartoner
Bundle
Packer
Advanced
Bundle
Station
Datamatrix Station
or Bottle Serialization Station
Pilot
Database
Pilot
Site
Manager
Pilot
LIne
Manager
Shipping
Case
Packer
Shipping
Case
Packer
Site 3
Site 1
Datamatrix Station XMV
Safety Through Traceability
Marking, Verification and Sorting
A Fully Integrated Compact System
The Datamatrix Station XMV (DMS XMV)
minimizes the potential risks in pharmaceuti-
cal packaging through Track & Trace mea-
sures and adherence to guidelines. The DMS
XMV provides coding and serial number
coding to meet product traceability in com-
pliance with the EU Directive, USA ePedigree
and other worldwide track and trace require-
ments. The product codes and variable data
printed on the folding cartons are verified
via cameras for accuracy and quality. The
concurrent protocols meet GMP requirements
and quality assessments.
The DMS XMV system can be easily integrated
into new production lines or can be added to
upgrade existing lines providing a full range
of functions and user-friendly operation.



















